Physiological research on oxidative fermentation and its industrial use
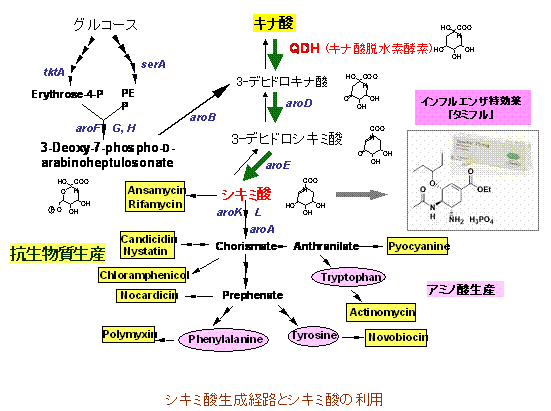
Recently, I have been focusing on research on 5-ketogluconic acid (5KGA) and shikimic acid (SKA) production systems.All of these are pharmaceutical materials that are difficult to mass-produce and are becoming a social problem, even though their uses are regarded as important.Although 5KGA is a useful chemical material in itself, it has attracted attention as a synthetic intermediate for tartrate, xylitol, and polyhydroxypolymers, and many researchers are trying fermentative production using acetic acid bacteria and related microorganisms.On the other hand, SKA, which is attracting attention not only as a lot of antibiotics but also as a raw material for Tamiflu, is currently supplied by the extraction method from the fruit of illicium anisatum and the chemical synthesis method, but there is a serious shortage of illicium anisatum. In addition, due to the difficulty of chemical synthesis of SKA, which contains three points, it is extremely difficult to supply it in large quantities.Regarding 5KGA production, overseas groups have reported a fermentation production method using genetic engineering techniques for acetobacter, but the problem is the growth disorder of the bacteria induced by the introduction of mutations.In this research, based on our research on "physiology of oxidative fermentation" and "molecular properties of oxidative fermentation enzyme" that we have developed over many years, we have taken an approach to effectively increase 5KGA production by acetic acid bacteria. We are getting unrivaled results from our group.On the other hand, regarding the production of shikimic acid, genetic engineering technology that causes Escherichia coli to produce SKA is currently being proposed. Since this system is synthesized from the first substance, glucose, via a multi-step metabolic pathway, low conversion efficiency is a major obstacle to mass production.Instead of glucose, we aim to synthesize intermediates using an efficient conversion system called oxidative fermentation from quinic acid, which is more direct, and convert them to SKA with a simple metabolic system.That is, intermediate 3-dehydroshikimic acid (DSA) is produced in high yield by directly reacting the acetic acid bacterium cell membrane fraction with quinic acid, and dehydrogenase shikimic acid is used to convert this DSA to SKA. Enzyme (SKDH) and glucose dehydrogenase (GDH) required for NADPH regeneration system are isolated and purified from acetic acid bacteria cytoplasm, respectively, and DSA is added with those purified enzymes and NADPH required for reaction, and excess glucose is present. Here's how to react below.It has become clear that this method can produce SKA with a conversion rate of almost 100%.
So far, the production of various types of extracellular polysaccharides has been reported from various microorganisms, and they are used as food additives.Until now, the production of various types of extracellular polysaccharides has been reported from various acetic acid bacteria, but the polysaccharides involved in the suspension of acetic acid bacteria on the medium surface are a type of Acetobacter acetic acid bacteria. It was only reported by Gluconacetobacter xylinus.This bacterium is known to produce cellulose (Cellulose) as a polysaccharide attached to the surface of the bacterium.We have clarified that the biofilm formed by Acetobacter acetobacter is a heteropolysaccharide having a different composition depending on a novel strain different from cellulose, and has established a purification method for it.For example, the Acetobacter aceti biofilm is a polysaccharide consisting of glucose and rhamnose, and the Acetobacter tropicalis SKU100 strain biofilm is a polysaccharide consisting of glucose, rhamnose and galactose.The heteropolysaccharides that form the membrane of these acetic acid bacteria are expected to have immunostimulatory ability, and are considered to be novel polysaccharides that are highly likely to be used as pharmaceuticals or food additives.In particular, acetobacter is a bacterium that has been used in the production of vinegar, and its safety as a food has been empirically guaranteed for thousands of years, so it has very advantageous properties for use in food. It can be said.
<Research achievements related to this research subject(After 2000)>
1)O. Adachi, Y. Fujii, Y. Ano, D. Moonmangmee, H. Toyama, E. Shinagawa, G. Theeragool, N. Lotong & K. Matsushita: Membrane-bound sugar alcohol dehydrogenase in acetic acid bacteria catalyzes L-ribulose formation and NAD-dependent ribitol dehydrogenase is independent of the oxidative fermentation; Biosci. Biotechnol. Biochem., 65, 115-125 (2001)
2)O. Adachi, Y. Fujii, M.F. Ghaly, H. Toyama, E. Shinagawa, & K. Matsushita: Membrane-bound quinoprotein D-arabitol dehydrogenase of Gluconobacter suboxydans IFO 3257: A versatile enzyme for the oxidative fermentation of various ketoses; Biosci. Biotechnol. Biochem., 65, 2755-2762 (2001)
3)D. Moonmangmee, Y. Fujii, H. Toyama, G. Theeragool, N. Lotong, K. Matsushita & O. Adachi: Purification and characterization of membrane-bound quinoprotein cyclic alcohol dehydrogenase from Gluconobacter frateurii CHM 9; Biosci. Biotechnol. Biochem., 65, 2763-2772 (2001)
4)E. Shinagawa, T. Fujishima, D. Moonmangmee, G. Theeragool, H. Toyama, K. Matsushita & O. Adachi: Purification and characterization of membrane-bound malate dehydrogenase from Acetobacter sp. SKU 14; Biosci. Biotechnol. Biochem., 66, 298-306 (2002)
5)D. Moonmangmee, O. Adachi, E. Shinagawa, H. Toyama, G. Theeragool, N. Lotong, & K. Matsushita: L-Erythrulose production by oxidative fermentation is calatalyzed by PQQ-containing membrane-bound dehydrogenase; Biosci. Biotechnol. Biochem., 66, 307-318 (2002)
6)S. Moonmangmee, H. Toyama, O. Adachi, G. Theeragool, N. Lotong, K. Matsushita: Purification and Characterization of a Novel Polysaccharide Involved in the Pellicle Produced by Thermotolerant Acetobacter Strain; Biosci. Biotechnol. Biochem., 65, 777-783 (2002)
7)K. Matsushita, Y. Fujii, Y. Ano, H. Toyama, M. Shinjoh, N. Tomiyama, T. Miyazaki, T. Sugisawa, T. Hoshsino, O. Adachi: 5-Keto-D-gluconate Production is Catalyzed by a Quinoprotein Glycerol Dehydrogenase, Major Polyol Dehydrogenase, in Gluconobacter sp. Appl. Environ. Microbiol., 69, 1959-1966 (2003)
8) O. Adachi, N. Yoshihara, S. Tanasupawat, H. Toyama, & K. Matsushita: Purification and Characterization of Membrane-bound Quinoprotein Quinate Dehydrogenase; Biosci. Biotechnol. Biochem., 67, 2115-2123 (2003)
9)O. Adachi, S. Tanasupawat, N. Yoshihara, H. Toyama, & K. Matsushita: 3-Dehydroquinate Production by Oxidative Fermentation and Further Conversion of 3-Dehydroquinate to the Intermediates in the Shikimate Pathway; Biosci. Biotechnol. Biochem., 67, 2124-2131 (2003)
10)D. Moonmangmee1 , O. Adachi, H. Toyama and K. Matsushita: d-Hexosaminate production by oxidative fermentation. Appl. Microbiol. Biotechnol. 66 (3), 253-258 (2004)
11)S. Vangnai, H. Toyama, W. De-eknamkul, N. Yoshihara, O. Adachi, K. Matsushita: Quinate oxidation in Gluconobacter oxydans IFO3244: purification and characterization of quinoprotein quinate dehydrogenase, FEMS Microbiol. Lett. 241, 157-162 (2004)
12) E. Shinagawa, H. Toyama, K. Matsushita, P. Tuitemwong, G Theeragool, and O. Adachi: A novel type of formaldehyde-oxidizing enzyme from the membrane of Acetobacter sp. SKU 14. Biosci. Biotechnol. Biochem. 70, 850-857 (2006)
13)O. Adachi, Y. Ano, H. Toyama, and K. Matsushita: High shikimate production from quinate with two enzymatic systems of acetic acid bacteria. Biosci. Biotechnol. Biochem., 70, 2579-2582 (2006)
14)O. Adachi, Y. Ano, H. Toyama, and K. Matsushita: Purification and characterization of NADP-dependent shikimate dehydrogenase from Gluconobacter oxydans IFO3244 and its application to enzymatic shikimate production. Biosci. Biotechnol. Biochem., 70, 2786-2789 (2006)
15)O. Adachi, Y. Ano, H. Toyama, and K. Matsushita: Enzymatic Preparation of metabolic intermediates, 3-dehydroquinate and 3-dehydroshikimate, in the shikimate pathway. Biosci. Biotechnol. Biochem., 70, 3081-3083 (2006)
<本研究課題に関連する科学研究費およびその他研究助成(2000年以降)>
・科研費・基盤 C:外山博英:グルコノバクター属酢酸菌のソルビトール代謝工学(平成18〜19年度)
・科研費・基盤 C:松下一信:酢酸菌キノプロテイン・グリセロール脱水素酵素と5-ケトグルコン酸生成(平成16〜17年度)
・DSM Nutritional Products AG ・共同研究:松下一信:Improvement of Oxidative Fermentations Using Gluconobacter Bacteria(平成18年4月1日〜20年3月31日)
・宇部興産・共同研究:松下一信:微生物が生産する多糖類利用技術(平成18年度)
・財団法人医薬資源研究振興会研究助成:松下一信:キナ酸を原料とするシキミ酸の高効率な新規製造法の開発(平成18年度)
・山口大学ベンチャービジネスラボラトリー(YU-VBL)研究支援:松下一信(代表);有用医薬素材である5-ケトグルコン酸及びシキミ酸高生産菌の開発(平成17〜18年度)
・野田産研研究助成:外山博英:5-ケトグルコン酸生成の安定な生産のための微生物生化学的研究(平成17年度)
・長瀬産業研究助成金:松下一信:Acetobacter 属酢酸菌の菌膜形成に関与する新規多糖の発見とその菌膜形成能制御に関する生化学的及び分子生物学的研究(平成14年4月〜15年3月)
・山口大学競争的教育研究重点化経費:松下一信:ポストゲノム対応型新規産業微生物学遺伝子機能を制御する微生物産業の新展開(14年度)