|
|
1-アザアズレンは非交互系含窒素縮合複素環であり、非ベンゼン系とも呼ばれます。一般に、通常知られている交互系化合物(ベンゼン系)とは性質が大きく異なります。そのため、有機化学の常識が通用しない場合もあります。しかし、1-アザアズレンの反応性を理解し、その性質をうまく利用しながら新たな分子を構築していく事に日々チャレンジしています!!
| 最近の研究から(Heterocycles, 2012, 84(1), 461-472.) |
| 1-アザアズレン誘導体から鈴木カップリング反応とアルドール縮合を利用して、4環性縮合複素環の合成に成功しました。この2つの生成物は構造異性体ですが、色が全く異なります。X線結晶構造解析や計算化学を用いて調べてみると、共鳴の様子が異なる事が分かりました。なお、橙色の方は、HeLa S3細胞 (腫瘍細胞)に対する細胞毒性(抗腫瘍性)があることも分かりました。 |
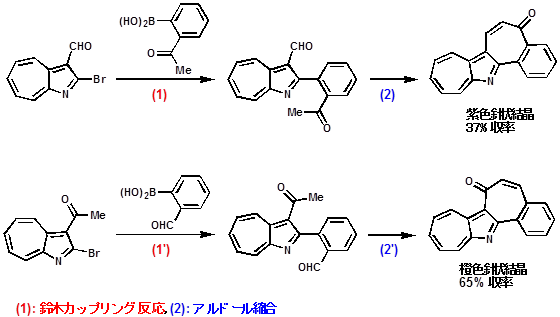 |
1.“The cycloaddition of 2-phenylamino-1-azaazulene with diphenylacetylene using palladium catalytic systems” Fujii, H.; Oka, S.; Nakamura, I.; Kawai, Y.; Ikeda, R.; Konakahara, T.; Abe, N.,Heterocycles, 2015, 90(1), 715-722.
2.“Synthesis of novel benzofuran fused 1-azaazulene derivative by tandem intermolecular Suzuki coupling/intramolecular Buchwald-Hartwig type coupling”Fujii, H.; Sanada, K.; Kawai, Y.; Ikeda, R.; Konakahara, T.; Abe, N.,Heterocycles, 2014, 80(1), 463-474.
3. “Synthesis and complexation of bis(1-azaazulen-2-yl)amines and bis(1-azaazulen-2-yl) sulfides” Yoshioka, E.; Koizumi, K.; Nakashima, K.; Fujii, H.i; Murafuji, T.; Gunji, T.; Abe, N., Heterocycles, 2012, 85(7), 1683-1695.
4. “Synthesis of benzotropone-annulated 1-azaazulenes and related compounds by Suzuki-Miyaura coupling/aldol condensation cascade reaction and evaluations of their cytotoxic activity against HeLa S3 cells” Nakatani, M.; Fujii, H.; Murafuji, T.; Gunji, T.; Ikeda, R.; Konakahara, T.; Abe, N., Heterocycles, 2012, 84(1), 461-472.
5. “Synthesis of 12,12a-dihydro-7,12a-diazacyclohepta[cd]benz[g]azulen-12-one (cyclohepta[mn]pyrrolo[2,1-c][1,4]benzodiazepin-12-one) and evaluation of cytotoxic activity against HeLa S3 cells” Yoshioka, E.; Fujii, H.; Murafuji, T.; Ikeda, R.; Konakahara, T.; Gunji, T.; Abe, N., Heterocycles, 2011, 83(6), 1409-1415.
6. “The reaction of [(1-azaazulen-2-yl)imino]phosphorane with arylaldehydes: formation of bis[(1-azaazulen-2-yl)amino](aryl)methanes” Fujii, H.; Nagamatsu, K.; Gunji, T.; Murafuji, T.; Abe, N., Heterocycles, 2010, 81(11), 2625-2633.
7. “Accumulation of lipid peroxide-derived, toxic α,β-unsaturated aldehydes (E)-2-pentenal, acrolein and (E)-2-hexenal in leaves under photoinhibitory illumination” Mano, J.; Tokushige, K.; Mizoguchi, H.; Fujii, H.; Khorobrykh, Sergey, Plant Biotechnology (Tokyo, Japan), 2010, 27(2), 193-197.
8. “Synthesis and some reactions of 11-azacyclohept[a]azulen-3(3H)-ones and evaluation of their cytotoxic activity against HeLa S3 cells” Ariyoshi, T.; Yoshinaga, K.; Koizumi, K.; Fujii, H.; Ikeda, R.; Konakahara, T.; Abe, N., Heterocycles, 2010, 80(1), 427-437.
9. “Heteroarylamination and heteroarylsulfidation of 2-chloro-1-azaazulenes” Yoshioka, E.; Koizumi, K.; Yamazaki, S.; Fujii, H.; Abe, N., Heterocycles, 2009, 78(12), 3065-3072.
10. “Palladium-catalyzed heteroarylamination of ethyl 2-chloro-1-azaazulene-3-carboxylate and annulation of heteroarylamino-1-azaazulenes” Koizumi, K.; Shimabara, K.; Takemoto, A.; Yamazaki, S.; Yamauchi, N.; Fujii, H.; Kurosawa, M.; Konakahara, T.; Abe, N., Heterocycles, 2009, 79, 319-324.
11. “Synthesis of [poly(2-pyridyl)-substituted]-1-azaazulenes” Ariyoshi, T.; Noda, T.; Watarai, S.; Tagashira, S.; Murakami, Y.; Fujii, H.; Abe, N., Heterocycles, 2009, 77(1), 565-574.
12. “Facile synthesis of (2-benzimidazolyl)-1-azaazulenes, (2-benzothiazolyl)-1-azaazulenes, and related compounds and evaluation of their anticancer in vitro activity” Yamauchi, N.; Fujii, H.; Kakehi, A.; Shiro, M.; Kurosawa, M.; Konakahara, T.; Abe, N., Heterocycles, 2008, 76(1), 617-634.
13. “Synthesis and reactions of 3-ethynyl-2-(triphenylphosphoimino)-1-azaazulenes” Abe, N.; Nagamatsu, K.; Ariyoshi, T.; Fujii, H.; Murakami, Y.; Tagashira, S.; Kakehi, A., Heterocycles, 2007, 74, 309-320.
14. “Syntheses of 2-formyl-, 2,3-diformyl- and 8-formyl-1-azaazulenes” Koizumi, K.; Miyake, C.; Ariyoshi, T.; Umeda, K.; Yamauchi, N.; Tagashira, S.; Murakami, Y.; Fujii, H.; Abe, N., Heterocycles, 2007, 73, 325-339.
15. “Synthesis of aryl conjugated (1-azaazulenyl)acetylenes and facile synthesis of thiophene fused 1-azaazulenes” Abe, N.; Harada, Y.; Imachi, Y.; Fujii, H.; Kakehi, A.; Shiro, M., Heterocycles, 2007, 72, 459-468.
16. “Cycloaddition reactions of 1-aza- and 1,3-diazaazulenium 1-methylides” Fujii, H.; Kawano, I.; Iwafuji, K.; Sawae, Y.; Nagamatsu, K.; Abe, N., Heterocycles, 2006, 70, 207-221.
17. “Reactions of 8-(triphenylphosphoimino)quinoline with aryl aldehydes and aryl isocyanates: formation of 2-aryl-4H-imidazo[4,5,1-ij]quinolines and related systems” Nagamatsu, K.; Akiyoshi, E.; Ito, H.; Fujii, H.; Kakehi, A.; Abe, N., Heterocycles, 2006, 69, 167-178.
18. “Effective methods for introducing some aryl and heteroaryl substituent onto 1-azaazulene nuclei” Abe, N.; Tanaka, M.; Maeda, T.; Fujii, H.; Kakehi, A., Heterocycles, 2005, 66, 229-240.
19. “Reactions of 2-triphenylphosphoimino-1-azaazulenes with aryl isocyanates and aryl isothiocyanates” Nagamatsu, K.; Serita, A.; Zeng, J.-H.; Fujii, H.; Abe, N.; Kakehi, A., Heterocycles, 2006, 67(1), 337-351.
20. “Reactions of 3-phenyl-8-triphenylphosphoimino-1-azaazulene with aryl isocyanate, aryl isothiocyanate, and carbon disulfide” Nagamatsu, K.; Fujii, H.; Abe, N.; Kakehi, A., Heterocycles, 2004, 64, 291-303.
|
|
|

