【Original Articles】
- Synthesis of 1‐amino‐3‐aryl Naphthalenes from Bis(Perfluoroalkanesulfonyl)Imide with 1,2‐diethynylbenzenes
Kawamoto, T.; Yamasaki, T.; Ikazaki, T.; Matsubara, H.; Kamimura, A.
Asian J. Org. Chem. 2024, e202400035. https://doi.org/10.1002/ajoc.202400035
ChemRxiv https://doi.org/10.26434/chemrxiv-2024-lwd3h
- Boosting Sulfonylation Reactions between Vinyl Triflates and Alkenes toward β-Ketosulfones Using Aqueous Sulfurous Acid.
Kawamoto, T.; Takeda, Y.; Kawabata, T.; Kamimura, A.
Synthesis 2024. https://doi.org/10.1055/a-2295-8117
- 2-Phenylsulfanylhydroquinone Dimer Mono-Quinone Derivative as a New Fluorescence Dye Responding to Reductive Conditions.
Kamimura, A.; Abe, K.; Kawamoto, T.
Arkivoc 2024, 202312086. https://doi.org/10.24820/ARK.5550190.P012.086
- Radical Cascade Reaction in Heterocyclic Synthesis: Formation of Bicyclic 2,3‐dihydrostannoles and Other Heterocyclic Compounds from Amide‐type 1,6‐Enyne Compounds.
Kamimura, A.; Kohno, K.; Ishido, K.; Kamohara, N.; Kawamoto, T.
Asian J. Org. Chem. 2023, 12, e202300223. https://doi.org/10.1002/ajoc.202300223
- Preparation and Hydrophilicity/Lipophilicity of Solubility-Switchable Ionic Liquids.
Kamimura, A.; Yanagisawa, K.; Kaneko, N.; Kawamoto, T.; Fujii, K.
ACS Omega 2022, 7, 48540–48554. https://doi.org/10.1021/acsomega.2c06998
- Vicinal Difunctionalization of Alkenes Using Vinyl Triflates Leading to γ-Trifluoromethylated Ketones
Kawamoto, T.; Kawabata, T.; Noguhi, K.; Kamimura, A.
Org. Lett. 2022, 24, 324–327.
Https://Doi.Org/10.1021/Acs.Orglett.1c03988
- One-Pot Synthesis of CF3 ‑ Substituted Vinyl Trifluoromethanesulfonamides from Imines and Trifluoromethanesulfonic Anhydride
Kawamoto, T.; Ikawa, K.; Kamimura, A.
J. Org. Chem. 2021, 86, 15818–15824.
Https://Doi.Org/10.1021/Acs.Joc.1c01969
- Synthesis of 1-(1-Arylvinyl)Pyridin-2(1 H )-Ones from Ketones and 2-Fluoropyridine .
Kawamoto, T.; Ikeda, S.; Kamimura, A.
J. Org. Chem. 2021, 86, 13783–13789.
Https://Doi.Org/10.1021/Acs.Joc.1c01615
- Blacklight‐induced Hydroxylation of Arylboronic Acids Leading to Hydroxyarenes.
Kawamoto, T.; Ryu, I.
Helv. Chim. Acta 2021, 104, e2100102.
Https://Doi.Org/10.1002/Hlca.202100102
- Borane Evolution and Its Application to Organic Synthesis Using the Phase-Vanishing Method.
Soga, N.; Yoshiki, T.; Sato, A.; Kawamoto, T.; Ryu, I.; Matsubara, H.
Tetrahedron Lett. 2021, 69, 152977.
Https://Doi.Org/10.1016/J.Tetlet.2021.152977
- Development of Water Solubility of 2-Phenylsulfanylhydroquinone Dimer Dye.
Kamimura, A.; Umemoto, H.; Kawamoto, T.; Honda, T.
ACS Omega 2021, 6, 9254–9262.
Https://Doi.Org/10.1021/Acsomega.1c00703
- Redox-neutral Tetrafluoroethylation of Aryl Alkynes with 1,1,2,2-Tetrafluoroethane sulfonic acid leading to α-Tetrafluoroethylated Acetophenones
Kawamoto, T.; Noguchi, K.; Sasaki, R.; Takata, R.; Matsubara, H.; Kamimura, A.
Chem. Eur. J. 2021, 9529–9534.
Https://Doi.Org/10.1002/Chem.202100137
Selected as Frontispiece
Https://Doi.Org/10.1002/Chem.202183763
- Inverse Hydroboration Of Imines With NHC-Boranes Is Promoted By Diphenyl Disulfide And Visible Light.
Kawamoto, T.; Morioka, T.; Noguchi, K.; Curran, D. P.; Kamimura, A.
Org. Lett. 2021, 23, 1825–1828.
Https://Doi.Org/10.1021/Acs.Orglett.1c00230
- Hydrodecyanation of Secondary Alkyl Nitriles and Malononitriles to Alkanes using DiMeImd-BH3.
Kawamoto, T.; Oritani, K.; Kawabata, A.; Morioka, T.; Matsubara, H.; Kamimura, A.
J. Org. Chem. 2020, 85, 6137.
Http://Dx.Doi.Org/10.1021/Acs.Joc.0c00105
- Highly Cumulated Radical Cascade Reaction of aza-1,6-Enyenes: Stereoselective Synthesis of exo-Methylene Piperidines.
Kamimura, A.; Itaya, T.; Yoshinaga, T.; Nozawa, R.; Kawamoto, T.; Sumimoto, M.; Uno, H.
Eur. J. Org. Chem. 2020, 700.
Http://Dx.Doi.Org/10.1002/Ejoc.202000034
- 2‐Sulfanylhydroquinone Dimer as a Switchable Fluorescent Dye.
Kamimura, A.; Sakamoto, S.; Umemoto, H.; Kawamoto, T.; Sumimoto, M.
Chem. Eur. J. 2019, 25, 14081.
Http://Dx.Doi.Org/10.1002/Chem.201903436
Selected as Cover Feature
Http://Dx.Doi.Org/10.1002/Chem.201904089
- The regioselective trifluoromethylation of 1,3-bis(vinyl triflates) in the absence of external trifluoromethyl sources.
Kawamoto, T.; Sasaki, R.; Kamimura, A.; Matsubara, H.
J. Fluorine Chem. 2019, 221, 66–69.
Http://Dx.Doi.Org/10.1016/J.Jfluchem.2019.04.002
- Depolymerization of polyamide 6 in hydrophilic ionic liquids.
Kamimura, A.; Shiramatsu, Y.; Kawamoto, T.
Green Energy and Environment 2019, 4, 166-170.
Http://Dx.Doi.Org/10.1016/J.Gee.2019.01.002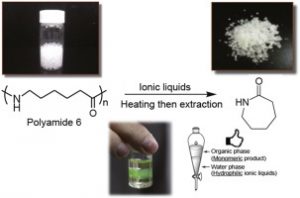
- Comparison of homofugality among alkyl groups attached to tin atom.
Kamimura, A.; Yoshinaga, T.; Miyazaki, K.; Kawamoto, T.
Heteroatom Chem. 2018, 2, e21469–7.
http://Dx.Doi.Org/10.1002/Hc.21469 - Asymmetric Michael Reaction of Aldehydes and Dicyanoalkenes Catalyzed by Diphenylprolinol Silyl Ether.
Hayashi, Y.; Kranidiotis-Hisatomi, N.; Sakamoto, D.; Oritani, K.; Kawamoto, T.; Kamimura, A.
Eur. J. Org. Chem. 2018, 6843–6847.
Http://Dx.Doi.Org/10.1002/Ejoc.201800831
- A Theoretical Study on Radical-Based Aminocarbonylation of Aryl Iodides.
Kawamoto, T.; Matsubara, H.; Fukuyama, T.; Ryu, I.
Chem. Lett. 2018, 47, 1169–1171.
Http://Dx.Doi.Org/10.1246/Cl.180599
- Theoretical Calculations for the 1,4-Hydrogen Shift of 1-Hydroxyallyl Radicals Leading to α-Keto Radicals; Prediction of Facilitation by 1-Amino and 3-Tin Substituents.
Matsubara, H.; Kawamoto, T.; Fukuyama, T.; Ryu, I.
Chem. Lett. 2018, 47, 1197–1199.
Http://Dx.Doi.Org/10.1246/Cl.180522
- Development of a microwave-assisted sustainable conversion of furfural hydrazones to functionalised phthalimides in ionic liquids.
Karaluka, V.; Murata, K.; Masuda, S.; Shiramatsu, Y.; Kawamoto, T.; Hailes, H. C.; Sheppard, T. D.; Kamimura, A.
RSC Adv. 2018, 8, 22617–22624.
Http://Dx.Doi.Org/10.1039/C8RA03895C
- Solubility-switchable ionic liquids: A control of hydrophilicity and hydrophobicity using a protective group.
Kamimura, A.; Shiramatsu, Y.; Murata, K.; Kawamoto, T.
Chem. Lett. 2018, 47, 1079–1081.
Http://Dx.Doi.Org/10.1246/Cl.180382
- Deltaarenes; novel macrocyclic molecules that are readily available from 1,4-benzoquinone and benzene dithiols.
Kamimura, A.; Watanabe, R.; Fukumitsu, T.; Ikeda, K.; Kawamoto, T.; Sumimoto, M.; Mori, S.; Uno, H.
Tetrahedron 2018, 74, 5303–5308.
Http://Dx.Doi.Org/10.1016/J.Tet.2018.04.070
- Thiol-Catalyzed Radical Decyanation of Aliphatic Nitriles with Sodium Borohydride
Kawamoto, T.; Oritani, K.; Curran, D. P.; Kamimura, A.
Org. Lett. 2018, 20, 2084–2087.
Https://Dx.Doi.Org/10.1021/Acs.Orglett.8b00626
- Tris (trimethylsilyl) silane-Mediated Reductive Decyanation and Cyano Transfer Reactions of Malononitriles
Kawamoto, T.; Shimaya, Y.; Curran, D. P.; Kamimura, A.
Chem. Lett. 2018, 47, 573–575.
Https://Doi.Org/10.1246/Cl.171231
- N-Heterocyclic Carbene Boryl Iodides Catalyze Insertion Reactions of N-Heterocyclic Carbene Boranes and Diazoesters
Allen, T.H.; Kawamoto, T.; Gardner, S.; Geib, S.J.; Curran, D.P.
Org. Lett. 2017, 19, 3680–3683.
Http://Dx.Doi.Org/10.1021/Acs.Orglett.7b01777
- An efficient and selective conversion of sorbitol in ionic liquids: Use of ion exchange resin as a solid acid catalyst
Kamimura, A.; Murata, K.; Kawamoto, T.
Tetrahedron Lett. 2017, 58, 3616–3618.
Http://Dx.Doi.Org/10.1016/J.Tetlet.2017.07.105
- Synthesis of α-Trifluoromethylated Ketones from Vinyl Triflates in the Absence of External Trifluoromethyl Sources
Kawamoto, T.; Sasaki, R.; Kamimura, A.
Angew. Chem. Int. Ed. 2017, 56, 1342–1345.
Http://Dx.Doi.Org/10.1002/Anie.201608591
Highlighted in Synfacts, 2017, 13. 0072.
Http://Dx.Doi.Org/10.1055/S-0036-1589791
- Asymmetric Synthesis of Bicyclic Nitrocyclopropanes from Primary Nitro Compounds and Stereoselective Formation of Tetrahydro-2H-cyclopenta[b]furans via Ring Expansion/Cyclization Reaction
Kamimura, A.; Moriyama, T.; Ito, Y.; Kawamoto, T.; Uno, H.
J. Org. Chem. 2016, 81, 4664–4681.
Http://Dx.Doi.Org/10.1021/Acs.Joc.6b00566
- 1,3-dimethylimidazoyl-2-ylidene borane
Gardner, S.; Kawamoto, T.; Curran, D.P.
Org. Synth. 2015, 92, 342–355.
Http://Dx.Doi.Org/10.15227/Orgsyn.092.0342
- A radical cascade reaction of aza-1,6-enyne compounds using allyltributyltin
Kamimura, A.; Miyazaki, K.; Kawamoto, T.; Uno, H.
Tetrahedron 2016, 72, 7722–7726.
Http://Dx.Doi.Org/10.1016/J.Tet.2016.04.078
- Oxidative synthesis of isoxazoline-N-oxide from optically active nitro alcohols
Moriyama, T.; Kawamoto, T.; Uno, H.; Kamimura, A.
Heterocycles 2016, 92, 1479–1489.
Http://Dx.Doi.Org/10.3987/COM-16-13497
- An oxidative cyclopropanation reaction of primary nitro compounds using Fe2O3
Moriyama, T.; Ito, Y.; Koyama, Y.; Kawamoto, T.; Kamimura, A.
Tetrahedron Lett. 2016, 57, 3127–3128.
Http://Dx.Doi.Org/10.1016/J.Tetlet.2016.06.013
- A regioselective double Stille coupling reaction of bicyclic stannolanes
Kamimura, A.; Tanaka, T.; So, M.; Itaya, T.; Matsuda, K.; Kawamoto, T.
Org. Biomol. Chem. 2016, 14, 8109–8122.
Http://Dx.Doi.Org/10.1039/C6ob01018k
- Synthesis of 1,3-Dialkylimidazol-2-ylidene Boranes from 1,3-Dialkylimidazolium Iodides and Sodium Borohydride
Gardner, S.; Kawamoto, T.; Curran, D.P.
J. Org. Chem. 2015, 80, 9794–9797.
Http://Dx.Doi.Org/10.1021/Acs.Joc.5b01682
- Photoinduced Aminocarbonylation of Aryl Iodides
Kawamoto, T.; Sato, A.; Ryu, I.
Chem. Eur. J. 2015, 21, 14764–14767.
Http://Dx.Doi.Org/10.1002/Chem.201503164
Selected as Hot Paper.
- Radical Reactions of N -Heterocyclic Carbene Boranes with Organic Nitriles: Cyanation of NHC-Boranes and Reductive Decyanation of Malononitriles
Kawamoto, T.; Geib, S.J.; Curran, D.P.
J. Am. Chem. Soc. 2015, 137, 8617–8622.
Http://Dx.Doi.Org/10.1021/Jacs.5b04677
- Borohydride-mediated radical addition reactions of organic iodides to electron-deficient alkenes
Kawamoto, T.; Uehara, S.; Hirao, H.; Fukuyama, T.; Matsubara, H.; Ryu, I.
J. Org. Chem. 2014, 79, 3999–4007.
Http://Dx.Doi.Org/10.1021/Jo500464q
- Cyanoborohydride-promoted radical arylation of benzene
Kawamoto, T.; Sato, A.; Ryu, I.
Org. Lett. 2014, 16, 2111–2113.
Http://Dx.Doi.Org/10.1021/Ol500614q
- A theoretical study on reduction of acyl radicals with borohydride anions
Kawamoto, T.; Matsubara, H.; Ryu, I.
Chem. Lett. 2014, 43, 1140–1142.
Http://Dx.Doi.Org/10.1246/Cl.140370
Selected as Editor’s Choice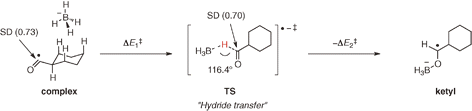
- Flow Giese reaction using cyanoborohydride as a radical mediator
Fukuyama, T.; Kawamoto, T.; Kobayashi, M.; Ryu, I.
Beilstein J. Org. Chem. 2013, 9, 1791–1796.
Http://Dx.Doi.Org/10.3762/Bjoc.9.208
- Efficient hydroxymethylation reactions of iodoarenes using CO and 1,3-dimethylimidazol-2-ylidene borane
Kawamoto, T.; Okada, T.; Curran, D.P.; Ryu, I.
Org. Lett. 2013, 15, 2144–2147.
Http://Dx.Doi.Org/10.1021/Ol4006294
- Radical addition of alkyl halides to formaldehyde in the presence of cyanoborohydride as a radical mediator. A new protocol for hydroxymethylation reaction
Kawamoto, T.; Fukuyama, T.; Ryu, I.
J. Am. Chem. Soc. 2012, 134, 875–877.
Http://Dx.Doi.Org/10.1021/Ja210585n
Highlighted in Synform, 2012, 5.
Http://Dx.Doi.Org/10.1055/S-0031-1290940
Highlighted in Nachrichten aus der Chemie, 2012, 60, 210.
Http://Dx.Doi.Org/10.1002/Nadc.201290114
- Stereocontrolled synthesis of substituted bicyclic ethers through oxy-Favorskii rearrangement: Total synthesis of (±)-communiol e
Kobayashi, S.; Kinoshita, T.; Kawamoto, T.; Wada, M.; Kuroda, H.; Masuyama, A.; Ryu, I.
J. Org. Chem. 2011, 76, 7096–7103.
Http://Dx.Doi.Org/10.1021/Jo201064h
Highlighted in Synfacts, 2011, 11. 1160.
Http://Dx.Doi.Org/10.1055/S-0031-1289273
- Thermal retro-aldol reaction using fluorous ether F-626 as a reaction medium
Fukuyama, T.; Kawamoto, T.; Okamura, T.; Denichoux, A.; Ryu, I.
Synlett 2010, 2193–2196.
Http://Dx.Doi.Org/10.1055/S-0030-1258501
- Black-light-induced radical/ionic hydroxymethylation of alkyl iodides with atmospheric co in the presence of tetrabutylammonium borohydride
Kobayashi, S.; Kawamoto, T.; Uehara, S.; Fukuyama, T.; Ryu, I.
Org. Lett. 2010, 12, 1548–1551.
Http://Dx.Doi.Org/10.1021/Ol1002847
【Reviews, Accounts, etc】
- Ionic Liquids for the Chemical Recycling of Polymeric Materials and Control of Their Solubility.
Kamimura, A.; Kawamoto, T.; Fujii, K.
Chem. Rec. 2023, e202200269. https://doi.org/10.1002/tcr.202200269
- New Directions in Radical Carbonylation Chemistry. Combination with Electron Catalysis, Photocatalysis and Ring-Opening.
Kawamoto, T.; Fukuyama, T.; Picard, B.; Ryu, I.
Chem. Commun. 2022, 7608–7617.
https://doi.org/10.1039/d2cc02700c
- ビニルトリフラートおよびその類縁体のラジカル反応
川本拓治,上村明男
有機合成化学協会誌,2020, 80, 554–562.
https://doi.org/10.5059/yukigoseikyokaishi.80.554
- Recent Advances in Radical Reactions of Vinyl Triflates and Their Derivatives.
Kawamoto, T.; Kamimura, A.
Synthesis 2022, 54, 2539–2547.
https://doi.org/10.1055/A-1765-7383/ID/JR000-3.
- Applications of Radical Carbonylation and Amine Addition Chemistry: 1,4-Hydrogen Transfer of 1‑Hydroxylallyl Radicals.
Matsubara, H.; Kawamoto, T.; Fukuyama, T.; Ryu, I.
Acc. Chem. Res. 2018, 51, 2023–2035.
Http://Dx.Doi.Org/10.1021/Acs.Accounts.8b00278
- Radical reactions of borohydrides
Kawamoto, T.; Ryu, I.
Org. Biomol. Chem. 2014, 12, 9733–9742.
Http://Dx.Doi.Org/10.1039/C4ob01784f
Selected as Inside front cover.
- Innovative carbonylation methods
Kawamoto, T.; Fukuyama, T.; Ryu, I.
有機合成化学協会誌 2014, 72, 493–505.
Http://Dx.Doi.Org/10.5059/Yukigoseikyokaishi.72.493
- Free radical-mediated hydroxymethylation using CO and HCHO
Kawamoto, T.; Ryu, I.
Chimia 2012, 66, 372–376.
Http://Dx.Doi.Org/10.2533/Chimia.2012.372