Laboratory of Applied Microbiology
Faculty of Agriculture, Yamaguchi University
Yoshida 1677-1, Yamaguchi, 753-8515 Japan
Tel: +81-83-933-5857; Fax: 5820; Email: obi(at)yamaguchi-u.ac.jp
日本語
Research Subject
Physiological research on oxidative fermentation and its industrial use
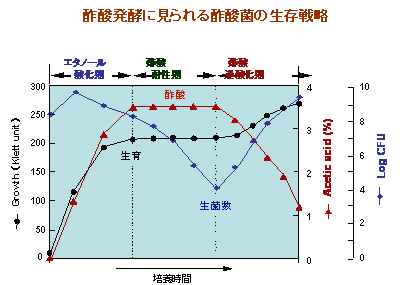
Acetobacter grows in coexistence and competition with various microorganisms in nectar and fruits containing high concentrations of sugar and alcohol and their rancid fruit wine, and has an extremely unique growth physiology in this growing environment.In other words, acetobacter can rapidly oxidatively convert certain sugars and alcohols into sugar acids (organic acids) that are difficult to use, and can accumulate in high concentrations in the medium.This reaction, called "oxidative fermentation," interferes with the use of sugars and alcohols by coexisting microbial species, and at the same time results in the accumulation of (harmful) organic acids and a low pH environment that are unsuitable for the survival of these microorganisms. But, under these conditions, the growth of acetobacter itself is not good either.However, acetobacter is relatively resistant to these organic acids and low pH, and has the ability to assimilate and utilize those sugar acids after a certain period of time to resume growth.Therefore, acetobacter shows a characteristic "Diauxie" (two-stage) growth. We humans have used this "selfish" survival strategy of acetobacter, oxidative fermentation, for a long time as vinegar brewing, and in recent years as sorbose fermentation and ketogluconic acid fermentation, but we do not know a useful oxidative fermentation system. However, there is a possibility that the oxidative fermentation system of acetobacter still exists.
This oxidative fermentation is mainly formed by the redox enzyme quinoprotein bound to the surface layer of the cell membrane and the respiratory chain terminal ubiquinol oxidase linked to it.This quinoprotein has pyrroloquinoline quinone as its prosthetic group, but its existence is almost limited to abg-proteobacteria, and the results support the new acquisition of ubiquinol oxidase in acetic acid bacteria. Has been obtained.
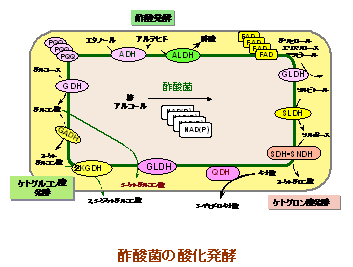
In this way, oxidative fermentation has come to be considered as an evolutionarily new system that provides the physiological functions characteristic of this acetic acid bacterium.
We are advancing research on the "fermentation physiology" of acetobacter, as well as applied research using acetobacter and its oxidative fermentation.
They include heat resistance of acetic acid fermentation (another issue), high efficiency of 5-ketogluconic acid fermentation, development of sikimic acid production system using dehydroshikimic acid fermentation, and biosensor polysaccharides produced in connection with acetic acid fermentation. Effective use, use of quinoprotein as a biosensor, etc.
⇒Latest efforts
Molecular mechanism of acetic acid fermentation
Among the above-mentioned "oxidative fermentation" of acetic acid bacteria, the most characteristic reaction is "acetic acid fermentation".
This acetic acid fermentation is based on the oxidation reaction of ethanol to acetic acid by the alcohol-oxidizing respiratory chain in the cell membrane.
Vinegar brewing using this reaction is an important fermentation industry that has been cultivated all over the world for a long time, but since ancient times it has been carried out by a static culture method that forms a thin biofilm on the surface of the medium in barrels and bottles. It has been received.
Today, aeration-stirring culture using tanks has been performed, but vinegar brewing by static culture is still being performed.
However, this acetic acid fermentation is extremely unstable, especially in static culture, making it difficult to stably produce high-quality products.
There are various factors involved in this instability of acetic acid fermentation, but in high temperatures such as in summer, static culture is not possible because biofilms are not formed.
In addition, a large amount of cooling water is required for tank culture.
In addition, the phenomenon that bacteria are killed by the produced acetic acid (loss of acetic acid resistance) and the phenomenon that the produced acetic acid is consumed (acetic acid peroxidation) frequently occur.
These phenomena make it difficult for small and medium-sized local companies that do not have advanced fermentation control systems to produce traditional vinegar rooted in the region.

On the other hand, in the tropical and subtropical regions, which are always under high temperature, acetic acid fermentation cannot be performed stably under high temperature, so the culture of using vinegar is not growing.
In order to address these issues, we play an important role in acetic acid fermentation, which is unique to Acetobacter genus acetic acid bacteria (1) Ethanol oxidation mechanism (acetic acid-producing ability),
(2) Elucidation of the mechanism of biofilm polysaccharide production (pellicle forming ability), (3) the mechanism of acetic acid resistance of this bacterium that survives the high concentration of acetic acid produced, and (4) the mechanism of acetic acid peroxidation harmful to acetic acid fermentation. At the same time, we are currently conducting research on the relationship between these factors and the culture temperature.
⇒Latest efforts
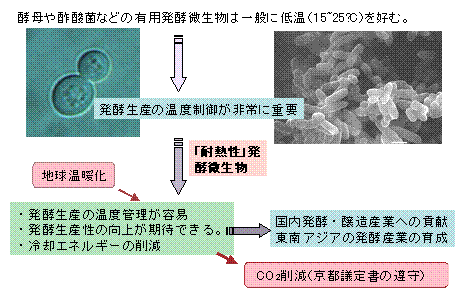
Heat-resistant fermenting microorganisms(Thermostable acetobacter)Project
Not only yeast and acetic acid bacteria, but also fermenting microorganisms generally used by humans prefer relatively low temperatures (15 to 25 ° C).
It is well known that an increase in fermentation temperature leads to a decrease in bacterial growth and further a decrease in fermentation productivity.
In particular, due to recent global warming, it is not uncommon for high temperatures of over 30 degrees Celsius to continue in the summer.
Moreover, today, the emergence of biotechnology in Southeast Asia is remarkable, and the domestic microbial industry is here.
It is becoming more common to start a new fermentation industry. Under these circumstances, relatively high temperature (30-40 ° C)
Breeding of microbial strains capable of normal fermentation production in Japan is considered to be of great value.
⇒Latest efforts
Study on the structure and function of quinoprotein dehydrogenase
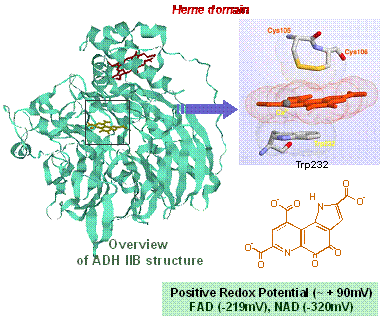
We used the third coenzyme of redox reaction, pyrroloquinoline quinone (PQQ), as methanol dehydrogenase (MDH).
Following the world's first cell membrane-bound alcohol dehydrogenase (type III ADH) and glucose dehydrogenase (mGDH) We have been elucidating the reaction mechanism of quinoproteins and conducting biochemical basis analysis.
In addition to type III ADH and mGDH since the early 1980s, soluble quinoprotein, water-soluble glucose dehydrogenase (sGDH),
Soluble quinohemoprotein alcohol dehydrogenase (type II ADH), which contains hem c as a prosthetic group in addition to PQQ,
Research on MDH of acetobacter has also started.
Membrane-bound quinoproteins from the late 1980s to the early 1990s
Involvement of quinoprotein in a technique that purifies and other respiratory chain components and reconstitutes them all into artificial membrane vesicles.
Completely elucidated the structure and function of the respiratory chain.
⇒Latest efforts
Kinohemoprotein ・ Structure of alcohol dehydrogenase and prosthetic group
Supplemental materials of the PhD thesis
design テンプレート